PERFORMANCE EVALUATION OF HYDROXYAPATITE PREPARED FROM EGGSHELLS IN CARBON DIOXIDE ADSORPTION
DOI:
https://doi.org/10.22452/mjs.vol43sp1.8Keywords:
Hydroxyapatite, eggshell, carbon dioxide adsorption, impregnation, deep eutectic solventAbstract
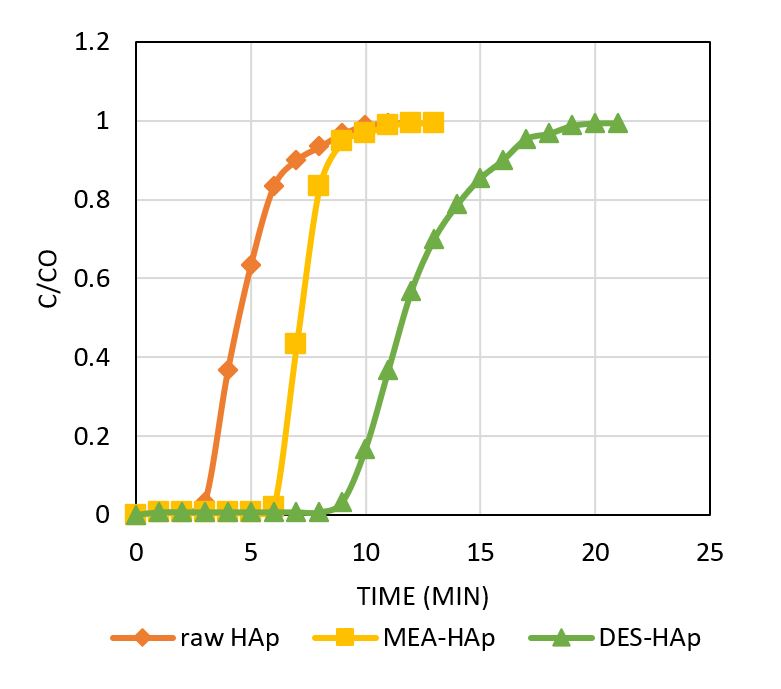
Eggshell waste is typically produced from daily poultry consumption and industrial applications. They are a rich source of calcium in the form of carbonates and oxides, recognised as excellent hydroxyapatite sources (HAp). To date, limited studies have highlighted the modification of HAp with impregnation. In the present study, HAp was prepared via the precipitation method, and further modification of HAp using monoethanolamine (MEA) and deep eutectic solvent, particularly choline chloride:urea (ChCl:U), were explored for carbon dioxide (CO2) capture. The morphological structures were studied using a scanning electron microscope, while properties were assessed using energy-dispersive X-ray spectroscopy techniques (SEM-EDX) and Brunauer-Emmett-Teller (BET). The CO2 adsorption performance using raw and impregnated HAp was also evaluated. By introducing the chemisorption process, the impregnated ChCl:U-HAp with irregular crystallite agglomerates demonstrated a higher adsorption capacity and longer breakthrough time than raw HAp and MEA-HAp. This study confirms the feasibility of using eggshells to produce HAp as an effective adsorbent in CO2 capture.
Downloads
References
Abatan OG., Alaba PA., Oni BA., Akpojevwe K., Efeovbokhan V. & Abnisa F. (2020). Performance of eggshells powder as an adsorbent for adsorption of hexavalent chromium and cadmium from wastewater. SN Applied Sciences 2(12): 1996. https://doi.org/10.1007/s42452-020-03866-w
Agbabiaka OG., Oladele IO., Akinwekomi AD., Adediran AA., Balogun AO., Olasunkanm OG. & Olayanju TMA. (2020). Effect of calcination temperature on hydroxyapatite developed from waste poultry eggshell. Scientific African 8: e00452. https://doi.org/10.1016/j.sciaf.2020.e00452
Ariyanto T., Masruroh K., Pambayun GYS., Mukti NIF, Cahyono RB, Prasetya A. & Prasetyo I. (2021). Improving the separation of CO2/CH4 using impregnation of deep eutectic solvents on porous carbon. ACS Omega 6(29): 19194–19201. https://doi.org/10.1021/acsomega.1c02545
Azmi AA. & Aziz MAA. (2019). Mesoporous adsorbent for CO2 capture application under mild condition: A review. Journal of Environmental Chemical Engineering 7(2): 103022. https://doi.org/10.1016/j.jece.2019.103022
Babaei M. & Haghtalab A. (2023). High-pressure CO2 solubility measurement in aqueous mixtures of (2-amino-2-methyl-1-propanol (AMP) and deep eutectic solvent (tetra butyl ammonium bromide 1: 3 ethylene glycol)) at various temperatures. Fluid Phase Equilibria 565: 113643. https://doi.org/10.1016/j.fluid.2022.113643
Chia SM., Chiong MC., Panpranot J. & Lee KM. (2023). Process optimisation on co-production of lignin and cellulose in deep eutectic solvent pretreatment of oil palm empty fruit bunch. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-023-05025-8
Das D. & Meikap BC. (2018). Comparison of adsorption capacity of mono-ethanolamine and di-ethanolamine impregnated activated carbon in a multi-staged fluidised bed reactor for carbon-dioxide capture. Fuel 224: 47–56. https://doi.org/10.1016/j.fuel.2018.03.090
Ghazali Z., Suhaili N., Tahari MNA., Yarmo MA., Hassan NH. & Othaman R. (2020). Impregnating deep eutectic solvent choline chloride:urea:polyethyleneimine onto mesoporous silica gel for carbon dioxide capture. Journal of Materials Research and Technology 9(3): 3249–3260. https://doi.org/10.1016/j.jmrt.2020.01.073
Ghosh S., Sarathi R. & Ramaprabhu S. (2019). Magnesium oxide modified nitrogen-doped porous carbon composite as an efficient candidate for high pressure carbon dioxide capture and methane storage. Journal of Colloid and Interface Science 539: 245–256. https://doi.org/10.1016/j.jcis.2018.12.063
Ho JY., Chang TT., Ho PC., Chang HK. & Chen PY. (2024). Fabrication of gyroid-structured, hierarchically-porous hydroxyapatite scaffolds by a dual-templating method. Materials Chemistry and Physics 314: 128854. https://doi.org/10.1016/j.matchemphys.2023.128854
Ho PH., Lofty V., Basta A. & Trens P. (2021). Designing microporous activated carbons from biomass for carbon dioxide adsorption at ambient temperature. A comparison between bagasse and rice by-products. Journal of Cleaner Production 294: 126260. https://doi.org/10.1016/j.jclepro.2021.126260
Karimi M., Zafanelli LFAS., Almeida JPP., Ströher GR., Rodrigues AE. & Silva JAC. (2020). Novel insights into activated carbon derived from municipal solid waste for CO2 uptake: Synthesis, adsorption isotherms and scale-up. Journal of Environmental Chemical Engineering 8(5): 104069. https://doi.org/10.1016/j.jece.2020.104069
Khalil S. (2018). Effects on surface area, intake capacity and regeneration of impregnated palm-shell activated carbon with monoethanolamide and 2-amino-2-methyl-1-propanol equipped for CO2 adsorption. Journal of Earth Science & Climatic Change 9(7): https://doi.org/10.4172/2157-7617.1000484
Khoshraftar Z. & Ghaemi A. (2022). Presence of activated carbon particles from waste walnut shell as a biosorbent in monoethanolamine (MEA) solution to enhance carbon dioxide absorption. Heliyon 8(1): e08689. https://doi.org/10.1016/j.heliyon.2021.e08689
Latiff AFA., Yee LS., Muhammad MS., Chuan LT & Basri H. (2022). Natural adsorbent made from eggshells for removal of chromium (VI) in water. Biointerface Research in Applied Chemistry 12(1): 518–528. https://doi.org/10.33263/BRIAC121.518528
Makoś P., Słupek E. & Małachowska A. (2020). Silica gel impregnated by deep eutectic solvents for adsorptive removal of BTEX from gas streams. Materials 13(8): 1894. https://doi.org/10.3390/ma13081894
Massayev S. & Lee KM. (2022). Evaluation of deep eutectic solvent pretreatment towards efficacy of enzymatic saccharification using multivariate analysis techniques. Journal of Cleaner Production 360: 132239. https://doi.org/10.1016/j.jclepro.2022.132239
Mignardi S., Archilletti L., Medeghini L. & De Vito C. (2020). Valorisation of eggshell biowaste for sustainable environmental remediation. Scientific Reports 10(1): 2436. https://doi.org/10.1038/s41598-020-59324-5
Raja Shahrom MS., Nordin AR. & Wilfred CD. (2019). The improvement of activated carbon as CO2 adsorbent with supported amine functionalised ionic liquids. Journal of Environmental Chemical Engineering 7(5): 103319. https://doi.org/10.1016/j.jece.2019.103319
Rattanaphan S., Rungrotmongkol T. & Kongsune P. (2020). Biogas improving by adsorption of CO2 on modified waste tea activated carbon. Renewable Energy 145: 622–631. https://doi.org/10.1016/j.renene.2019.05.104
Tangboriboon N., Suttiprapar J., Changkhamchom S. & Sirivat A. (2019). Alternative green preparation of mesoporous calcium hydroxyapatite by chemical reaction of eggshell and phosphoric acid. International Journal of Applied Ceramic Technology 16(5): 1989–1997. https://doi.org/10.1111/ijac.13241
Tizo MS., Blanco LAV., Cagas ACQ., Dela Cruz BRB., Encoy JC., Gunting JV., Arazo RO. & Mabayo VIF. (2018). Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustainable Environment Research 28(6): 326–332. https://doi.org/10.1016/j.serj.2018.09.002
Trivedi TJ., Lee JH., Lee HJ., Jeong YK. & Choi JW. (2016). Deep eutectic solvents as attractive media for CO2 capture. Green Chemistry 18(9): 2834–2842. https://doi.org/10.1039/C5GC02319J
Unugul T. & Nigiz FU. (2020). Preparation and characterisation an active carbon adsorbent from waste mandarin peel and determination of adsorption behavior on removal of synthetic dye solutions. Water, Air, & Soil Pollution 231(11): 538. https://doi.org/10.1007/s11270-020-04903-5
Zhao H., Luo X., Zhang H., Sun N., Wei W. & Sun Y. (2018). Carbon‐based adsorbents for post‐combustion capture: A review. Greenhouse Gases: Science and Technology 8(1): 11–36. https://doi.org/10.1002/ghg.1758
Zohdi S., Anbia M. & Salehi S. (2019). Improved CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity in novel hollow silica particles by modification with multi-walled carbon nanotubes containing amine groups. Polyhedron 166: 175–185. https://doi.org/10.1016/j.poly.2019.04.001
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





