SYNTHESIS AND CHARACTERIZATION OF 2-(METHYLAMINO)ETHANOL-BASED DEEP EUTECTIC SOLVENTS FOR CO2 CAPTURE
DOI:
https://doi.org/10.22452/mjs.vol43sp1.5Keywords:
Deep eutectic solvents, 2 (methylamino)ethanol, choline hydroxide, physiochemical properties, CO2 solubilityAbstract
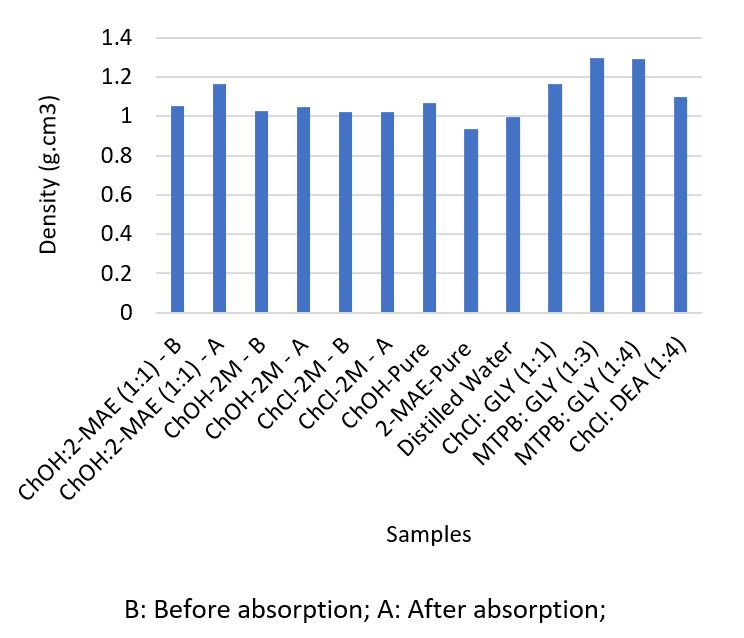
In recent years, deep eutectic solvents (DESs) have attracted the interest of many researchers for application in a wide range of industrial and scientific fields, including carbon dioxide (CO2) capture. DESs exhibit favourable solvent properties for CO2 removal applications; hence, they have become promising alternatives to common amine solutions and ionic liquids (ILs). In this context, a novel DES was synthesised by mixing 2 (methylamino)ethanol (2-MAE) as a hydrogen bond donor with choline hydroxide (ChOH) as a hydrogen bond acceptor with a molar ratio of ChOH:2-MAE of 1:1. The solubility of CO2 in the prepared systems was determined and characterised before and after CO2 absorption by measuring their physicochemical properties (density and viscosity) and analysing their FTIR spectra. The results showed that the DES and 2M DES aqueous solutions exhibited CO2 absorption capacities comparable to those of other reported DESs. These physicochemical properties were comparable to those reported in the literature. Besides, the FTIR analysis of the studies systems after absorption indicates the formation of carbamate.
Downloads
References
Agency, E. P. (2021, April 14). Overview of Greenhouse Gases. Retrieved from https://www.epa.gov/ghgemissions/overview-greenhouse-gases
Anderson, T. R., Hawkins, E., & Jones, P. D. (2016). CO2, the greenhouse effect and global warming: from the pioneering work of Arrhenius and Callendar to today's Earth System Models. Endeavour, 40(3), 178-187.
Chen, C.-C., Huang, Y.-H., Hung, S.-M., Chen, C., Lin, C.-W., & Yang, H.-H. (2021). Hydrophobic deep eutectic solvents as attractive media for low-concentration hydrophobic VOC capture. Chemical Engineering Journal, 424, 130420.
Chen, P.-C., & Lin, S.-Z. (2018). Optimization in the Absorption and Desorption of CO2 Using Sodium Glycinate Solution. Applied Sciences, 8, 2041.
Dincer, I., & Abu-Rayash, A. (2020). Chapter 1 - Fundamental aspects of energy, environment, and sustainability. In I. Dincer & A. Abu-Rayash (Eds.), Energy Sustainability (pp. 1-18): Academic Press.
Ghaedi, H., Ayoub, M., Sufian, S., Shariff, A. M., Hailegiorgis, S. M., & Khan, S. N. J. J. o. M. L. (2017). CO2 capture with the help of Phosphonium-based deep eutectic solvents. 243, 564-571.
Hussin, F., Aroua, M. K., & Yusoff, R. (2021). Adsorption of CO2 on palm shell based activated carbon modified by deep eutectic solvent: Breakthrough adsorption study. Journal of Environmental Chemical Engineering, 9(4), 105333.
IEA, C. (2023). Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach, IEA, Paris. Retrieved from https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach
Kassim, M. A., Sairi, N. A., Yusoff, R., Alias, Y., & Aroua, M. K. (2016). Evaluation of 1-Butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide–Alkanolamine Sulfolane-Based System as Solvent for Absorption of Carbon Dioxide. Industrial & Engineering Chemistry Research, 55(29), 7992-8001.
Murshid, G., Mjalli, F. S., Naser, J., Al-Zakwani, S., & Hayyan, A. (2019). Novel diethanolamine based deep eutectic mixtures for carbon dioxide (CO2) capture: synthesis and characterisation. Physics and Chemistry of Liquids, 57(4), 473-490.
Nanda, S., Reddy, S. N., Mitra, S. K., & Kozinski, J. A. (2016). The progressive routes for carbon capture and sequestration. 4(2), 99-122.
Nowosielski, B., Jamrógiewicz, M., Łuczak, J., Śmiechowski, M., & Warmińska, D. (2020). Experimental and predicted physicochemical properties of monopropanolamine-based deep eutectic solvents. Journal of Molecular Liquids, 309, 113110.
Pishro, K. A., Murshid, G., Mjalli, F. S., & Naser, J. (2020). Investigation of CO2 solubility in monoethanolamine hydrochloride based deep eutectic solvents and physical properties measurements. Chinese Journal of Chemical Engineering, 28(11), 2848-2856.
Sarmad, Shokat, & Nikjoo. (2020). Amine functionalized deep eutectic solvent for CO2 capture: Measurements and modeling. Journal of Molecular Liquids, 309, 113159.
Sarmad, S., Xie, Y., Mikkola, J.-P., & Ji, X. (2017). Screening of deep eutectic solvents (DESs) as green CO2 sorbents: from solubility to viscosity. New Journal of Chemistry, 41(1), 290-301.
Shahbaz, Bagh, & Mjalli. (2013). Prediction of refractive index and density of deep eutectic solvents using atomic contributions. Fluid Phase Equilibria, 354, 304-311.
Smith, E. L., Abbott, A. P., & Ryder, K. S. (2014). Deep Eutectic Solvents (DESs) and Their Applications. Chemical Reviews, 114(21), 11060-11082.
Suzuki, Y. (2018). Asymmetric Michael Addition Mediated by Chiral Ionic Liquids. Mini-reviews in organic chemistry, 15(3), 236-245.
Tomé, L. I. N., Baião, V., da Silva, W., & Brett, C. M. A. (2018). Deep eutectic solvents for the production and application of new materials. Applied Materials Today, 10, 30-50.
Wang, J., Cheng, H., Song, Z., Chen, L., Deng, L., & Qi, Z. (2019). Carbon Dioxide Solubility in Phosphonium-Based Deep Eutectic Solvents: An Experimental and Molecular Dynamics Study. Industrial & Engineering Chemistry Research, 58(37), 17514-17523.
Zhang, Q., De Oliveira Vigier, K., Royer, S., & Jérôme, F. (2012). Deep eutectic solvents: syntheses, properties and applications. Chemical Society Reviews, 41(21), 7108-7146.
Zubeir, L. F., Lacroix, M. H. M., Meuldijk, J., Kroon, M. C., & Kiss, A. A. (2018). Novel pressure and temperature swing processes for CO2 capture using low viscosity ionic liquids. Separation and Purification Technology, 204, 314-327.
Zurob, E., Cabezas, R., Villarroel, E., Rosas, N., Merlet, G., Quijada-Maldonado, E., . . . Plaza, A. (2020). Design of natural deep eutectic solvents for the ultrasound-assisted extraction of hydroxytyrosol from olive leaves supported by COSMO-RS. Separation and Purification Technology, 248, 117054.
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





