IMMOBILISATION OF COPPER (I) OXIDE/ZINC OXIDE NANOPARTICLES ON THE GAS DIFFUSION LAYER FOR CO2 REDUCTION REACTION APPLICATION
Main Article Content
Abstract
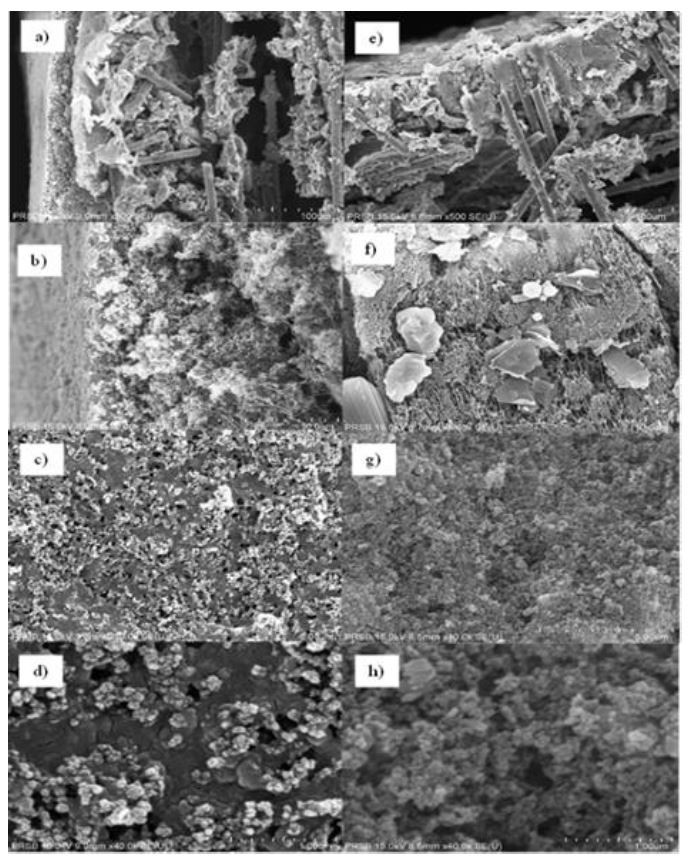
The electrochemical reduction of carbon dioxide (CO₂RR) represents a promising strategy for CO₂ mitigation, requiring highly efficient catalysts integrated into electrochemical devices to achieve high conversion rates and energy efficiencies for desired products. Establishing a gas diffusion electrode is crucial for practical applications of CO₂ electrochemical reduction reactions (CO₂RR). This study uses the air-spraying method to immobilise nano-catalysts onto a gas diffusion layer (GDL) with exceptional homogeneity. A composite of copper(I) oxide (Cu₂O) and zinc oxide (ZnO) nanoparticles in a 4:1 ratio was deposited onto the GDL. Surface morphology analysis revealed the successful immobilisation of cubic Cu₂O and hexagonal wurtzite ZnO with a uniform distribution, indicating potential improvements in CO₂RR performance. Contact angle measurements were conducted to assess surface hydrophobicity, comparing pristine GDL with Cu₂O/ZnO-based GDL. Although the contact angle on the surface of the Cu₂O/ZnO-based GDL slightly reduced from 143.69° to 134.82°, it maintained its hydrophobic nature. This reduction is attributed to Nafion, a binder in the catalyst ink mixture. The sustained high contact angle is crucial for the CO₂ reduction reaction process. X-ray diffraction (XRD) diffractograms of Cu₂O/ZnO-based GDL were compared with reference Cu₂O, ZnO, and bare GDL. The presence of all essential peaks confirms the successful immobilisation. The air-spraying technique effectively achieved a favourable distribution of active metals.
Downloads
Article Details
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).
References
Ahmad Zuhdi, M. F., Rahman, F. H., Shahjavan, H., Mas’od, M. A., Salihuddin, R. S., Zulkepli, N. A., Alias, A., Jalani, M. Y., & Yiin, T. K. (2021). Feasibility Study of Offshore Hybrid Technology for High CO2 Gas Field Monetization. Proceedings of International Petroleum Technology Conference, March, Virtual.
Albo, J., & Irabien, A. (2016). Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. In Journal of Catalysis 343: 232–239.
Burdyny, T., & Smith, W. A. (2019). CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy and Environmental Science 12(5): 1442–1453.
Chang, Q., Lee, J. H., Liu, Y., Xie, Z., Hwang, S., Marinkovic, N. S., Park, A. H. A., Kattel, S., & Chen, J. G. (2022). Electrochemical CO2Reduction Reaction over Cu Nanoparticles with Tunable Activity and Selectivity Mediated by Functional Groups in Polymeric Binder. JACS Au 2(1): 214–222.
Chen, X., Chen, J., Alghoraibi, N. M., Henckel, D. A., Zhang, R., Nwabara, U. O., Madsen, K. E., Kenis, P. J. A., Zimmerman, S. C., & Gewirth, A. A. (2021). Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nature Catalysis 4(1): 20–27.
Chu, M., Chen, C., Wu, Y., Yan, X., Jia, S., Feng, R., Wu, H., He, M., & Han, B. (2021). Enhanced CO2 electroreduction to ethylene via strong metal-support interaction. Green Energy and Environment 7(4): 792-798.
De Luna, P., Quintero-Bermudez, R., DInh, C. T., Ross, M. B., Bushuyev, O. S., Todorović, P., Regier, T., Kelley, S. O., Yang, P., & Sargent, E. H. (2018). Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nature Catalysis 1(2): 103–110.
Guzman, H., Zammillo, F., Roldan, D., Galleti, C., Russo, N., & Hernandez, S. (2021). Investigation of Gas Diffusion Electrode Systems for the Electrochemical CO2 Conversion. Catalysts 11(4): 482.
Hassan, H., Omar, N. F. N., Jalil, A. A. M. M., Salihuddin, R. S., & Shah, S. S. M. (2018). Gearing toward CCUS for CO2 reduction in Malaysia. Proceedings of Offshore Technology Conference Asia March, Kuala Lumpur.
Hoang, T. T. H., Verma, S., Ma, S., Fister, T. T., Timoshenko, J., Frenkel, A. I., Kenis, P. J. A., & Gewirth, A. A. (2018). Nanoporous Copper-Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. Journal of the American Chemical Society 140(17): 5791–5797.
Jhong, H. R. Q., Brushett, F. R., & Kenis, P. J. A. (2013). The effects of catalyst layer deposition methodology on electrode performance. Advanced Energy Materials 3(5): 589–599.
Jouny, M., Luc, W., & Jiao, F. (2018). General Techno-Economic Analysis of CO2 Electrolysis Systems. Industrial and Engineering Chemistry Research 57(6): 2165–2177.
Jung, H., Lee, S. Y., Lee, C. W., Cho, M. K., Won, D. H., Kim, C., Oh, H. S., Min, B. K., & Hwang, Y. J. (2019). Electrochemical Fragmentation of Cu 2 O Nanoparticles Enhancing Selective C-C Coupling from CO 2 Reduction Reaction. Journal of the American Chemical Society 141(11): 4624–4633.
Keerthiga, G., & Chetty, R. (2017). Electrochemical Reduction of Carbon Dioxide on Zinc-Modified Copper Electrodes. Journal of The Electrochemical Society 164(4): 164–169.
Kibria, M. G., Edwards, J. P., Gabardo, C. M., Dinh, C. T., Seifitokaldani, A., Sinton, D., & Sargent, E. H. (2019). Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Advanced Materials31(31): 1–24.
Kim, Dahee, Lee, S., Ocon, J. D., Jeong, B., Lee, J. K., & Lee, J. (2015). Insights into an autonomously formed oxygen-evacuated Cu2O electrode for the selective production of C2H4 from CO2. Physical Chemistry Chemical Physics 17(2): 824–830.
Kim, Dohyung, Kley, C. S., Li, Y., & Yang, P. (2017). Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proceedings of the National Academy of Sciences, 15 August, pp. 10560–10565 United States of America.
Kim, J., Choi, W., Park, joon woo, Kim, C., Kim, M., & Song, H. (2019). Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Production by Electrochemical Carbon Dioxide Reduction 141(17): 6986-6994.
Lee, M. Y., Park, K. T., Lee, W., Lim, H., Kwon, Y., & Kang, S. (2020). Current achievements and the future direction of electrochemical CO2 reduction: A short review. Critical Reviews in Environmental Science and Technology 50(8): 769–815.
Lee, S., Kim, D., & Lee, J. (2015). Electrocatalytic production of C3-C4 compounds by conversion of CO2 on a chloride-induced Bi-phasic Cu2O-Cu catalyst. Angewandte Chemie - International Edition 54(49): 14701–14705.
Liang, S., Altaf, N., Huang, L., Gao, Y., & Wang, Q. (2020). Electrolytic cell design for electrochemical CO2 reduction. Journal of CO2 Utilization 35: 90–105.
Lin, R., Guo, J., Li, X., Patel, P., & Seifitokaldani, A. (2020). Electrochemical reactors for CO2 conversion. In Catalysts 10(5): .
Mahamuni, P. P., Patil, P. M., Dhanavade, M. J., Badiger, M. V., Shadija, P. G., Lokhande, A. C., & Bohara, R. A. (2019). Using polyol chemistry for their antimicrobial and antibiofilm activity. Biochemistry and Biophysics Reports 17:71–80.
Merino-Garcia, I., Albo, J., & Irabien, A. (2018). Tailoring gas-phase CO2 electroreduction selectivity to hydrocarbons at Cu nanoparticles. Nanotechnology 29(1):014001.
Merino-Garcia, Ivan, Albo, J., Solla-Gullón, J., Montiel, V., & Irabien, A. (2019). Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. Journal of CO2 Utilization 31: 135–142.
Mok, D. H., Li, H., Zhang, G., Lee, C., Jiang, K., & Back, S. (2023). Data-driven discovery of electrocatalysts for CO2 reduction using active motifs-based machine learning. Nature Communications, 14(7303): 1-12.
Mowbray, B. A. W., Dvorak, D. J., Taherimakhsousi, N., & Berlinguette, C. P. (2021). How Catalyst Dispersion Solvents Affect CO2Electrolyzer Gas Diffusion Electrodes. Energy and Fuels 35(23): 19178–19184.
Qiao, J., Liu, Y., Hong, F., & Zhang, J. (2014). A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. In Chemical Society Reviews 43(2): 631-675.
Ramasamy, R.P. (2020). Membrane Electrode Assemblies, pp. 787–805, Elsevier B.V.
Reske, R., Mistry, H., Behafarid, F., Roldan Cuenya, B., & Strasser, P. (2014). Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. Journal of the American Chemical Society 136(19): 6978–6986.
Strijevskaya, A., Yamaguchi, A., Shoji, S., Ueda, S., Hashimoto, A., Wen, Y., Wardhana, A. C., Lee, J. E., Liu, M., Abe, H., & Miyauchi, M. (2023). Nanophase-Separated Copper-Zirconia Composites for Bifunctional Electrochemical CO2 Conversion to Formic Acid. ACS Applied Materials and Interfaces 15(19): 23299–23305.
Tang, Z., Nishiwaki, E., Fritz, K. E., Hanrath, T., & Suntivich, J. (2021). Cu(I) Reducibility Controls Ethylene vs Ethanol Selectivity on (100)-Textured Copper during Pulsed CO2Reduction. ACS Applied Materials and Interfaces 13(12): 14050–14055.
Yang, H., Chuai, H., Meng, Q., Wang, M., Zhang, S., & Ma, X. (2023). Copper-based bimetallic electrocatalysts for CO2 reduction: From mechanism understandings to product regulations. Materials Reports: Energy 3(1): 100174.
Yuan, L., Zeng, S., Zhang, X., Ji, X., & Zhang, S. (2023). Advances and challenges of electrolyzers for large-scale CO2 electroreduction. Materials Reports: Energy 3(1): 100177.
Zhang, J., Luo, W., & Zuttel, A. (2019). Self-supported copper-based gas diffusion electrodes for CO2 electrochemical reduction. Journal of Materials Chemistry A 46: 1–9.