HYDROTHERMAL SYNTHESIS OF NITROGEN-DOPED CQDS OF RUBUS NIVEUS LEAVES FOR FLUORESCENT pH SENSING AND PHOTOCATALYTIC APPLICATIONS
Main Article Content
Abstract
The development of a fluorescent pH sensor and the treatment of wastewater with nanoparticles are both critical topics. The variation in pH impacts the morphology and subsequent properties of the nanoparticles, which are used for their utilization in various fields and the second one gives a better treatment approach for industrial waste entities.
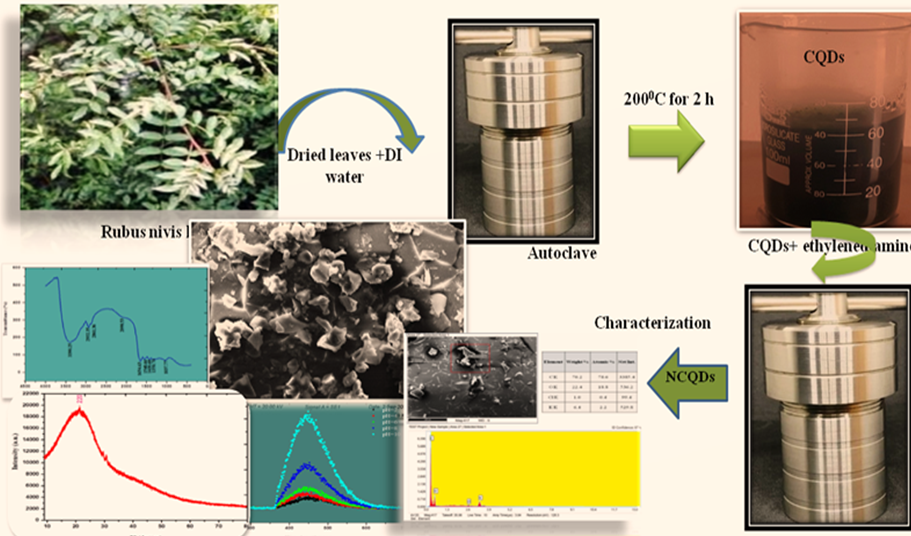
The present study examines the effects of these variables on the biologically produced nitrogen-doped carbon quantum dots (NCQDs). Hydrothermal process was performed for the synthesis of the same by using Rubus-niveus leaf extract as a precursor. The UV-Vis spectroscopy analysis shows absorption spectra in the wide range of 200 nm to 800 nm and shows prominent peaks at 236 nm and 392 nm, respectively. Further, the direct energy band gap of 3.65eV was analysed for NCQDs. The SEM image shows flower-shaped particles, and FT-IR analysis indicates the existence of amide, CHO, N-H, and C-N functional groups. XRD pattern showed that the surface morphology of NCQDs is amorphous in nature. A good response was found in the variation of fluorescence intensity with the pH values, confirming the possibility of NCQDs serving as pH sensors. Rhodamine-B (Rh-B) dye's reaction kinetics was revealed for the purpose of analyzing the potential of NCQDs for the degradation of commercial dyes and found to be followed by pseudo-first order kinetics with the correlation coefficient of 0.80.
Downloads
Article Details
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).
References
Arya S., Mahajan P., Mahajan S., Khosla A., Datt R., Gupta V., Young S.-J., Oruganti S.K.(2021). Review-Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol.10:23002. doi:10.1149/ 2162-8777/abe095
Bai Z. H., Chen R., Si P., Huang Y. J., Sun H. D., Kim D. H. (2013). Fluorescent pH Sensor Based on Ag@SiO2 Core-Shell Nanoparticle, ACS Applied Materials & Interfaces, 5(12): 5856-5860.
Barati A., Shamsipur M., Abdollahi H. (2016). Metal-ion-mediated fluorescent carbon dots for indirect detection of sulfide ions, Sens. Actuator B: Chem. 230: 289–297.
Basavaiah K., Tadesse A., Ramadevi D., Hagos M., Battu G.(2018). Facile green synthesis of fluorescent carbon quantum dots from citrus lemon juice for live cell imaging, Asian, J. Nanosci. Mater 1:36–46.
Blassan G., Thangaraj P., Sajeesh T., Saravanan S. (2014). Antitumor and wound healing properties of Rubus niveus Thunb root. J Environ Pathol Toxicol Oncol.33 (2): 145-158.
Bourlinos A.B., Karakassides M.A., Kouloumpis A., Gournis D., Bakandritsos A., Papagiannouli I. , Aloukos P., Couri S., Hola K., Zboril R., Krysmann M., Giannelis E.P. (2013). Synthesis, characterization and non-linear optical response of
organophilic carbon dots, Carbon 61: 640–646.
Carolan D., Rocks C., Padmanaban D.B., Maguire P., Svrcek V., Mariott D.(22017). Environmentally friendly nitrogen-doped carbon quantum dots for next generation solar cells Sustain. Energy Fuels 1:1611–1619.
Chan Y.H., Wu C.F., Ye F.M., Jin Y.H., Smith P.B., Chiu D.T. (2011). Development of Ultrabright Semiconducting Polymer Dots for Ratiometric pH Sensing, Anal. Chem. 83(4): 1448–1455.
Chang K., Zhu Q., Qi L., Guo M., Gao W., Gao Q. (2022). Synthesis and Properties of Nitrogen-Doped Carbon Quantum Dots Using Lactic Acid as Carbon Source. Materials. 15(2), 466. https://doi.org/10.3390/ma15020466
Cui L., Ren X., Sun M., Liu H., Xia L. (2021). Carbon Dots: Synthesis, Properties and Applications, Nanomaterials (Basel) 11: 3419.
Das B., Dadhich P., Pal P., Srivas P.K., Bankoti K., Dhara S.J. (2014). Carbon nanodots from date molasses: new nanolights for the in vitro scavenging of reactive oxygen species, Mater. Chem. B, 2: 6839–6847.
Dash U.N., Dash M.C. (1999). The effect of solvent polarity on the absorption and fluorescence of rhodamine dyes: a linear salvation energy relationship analysis. Journal of Luminescence 82(3): 169-180
Dennis A.M., Rhee W.J., Sotto D., Dublin S.N., Bao G. (2012). Quantum Dot–Fluorescent Protein FRET Probes for Sensing Intracellular pH, ACS Nano 6(4):2917–2924.
Dsouza, S. D. (20221). The importance of surface states in N-doped carbon quantum dots. Carbon, 183: 1–11. doi.org/10.1016/j.carbon.2021.06.088 0008
Du F., Zeng F., Ming Y., Wu S. (2013). Carbon dots-based fluorescent probes for sensitive and selective detection of iodide, Microchim. Acta 180:453-460.
Ge G. , Li L., Chen M., Wu X., Yang Y., Wang D., Zuo S., Zeng Z., Xiong W., Guo C. (2022). Green Synthesis of Nitrogen-Doped Carbon Dots from Fresh Tea Leaves for Selective Fe3+ Ions Detection and Cellular Imaging, Nanomaterials (Basel): 12: 986.
Han J.Y., Burgess K. (2010). Fluorescent Indicators for Intracellular pH, Chem. Rev. 110: 2709–2728.
Hoan B.T., Tam P.D., Pham V.H. (2019). Green Synthesis of Highly Luminescent Carbon Quantum Dots from Lemon Juice, J. Nanotechnol 2019: 1–9.
Jallel J. A., Pramod K. (201). Artful and multifaceted applications of carbon dot in biomedicine, J. Control. Release 269: 302–321.
Jin T., Sasaki A., Kinjo M., Miyazaki J. (2010). A quantum dot-based ratiometric pH sensor, Chem. Commun. 46: 2408–2410.
Khairol N.F., Sapawe N. (2018). Electrosynthesis of ZnO nanoparticles deposited onto egg shell for degradation of Congo red. Mater. Today Proc. 5: 21936–21939.
Khazan, A., Mohammadi A.H., Alimohammadi Z. (2017). Effects of solvent polarity on the absorption and fluorescence spectra of β-carotene in various solvents: A solvatochromic study. Journal of Luminescence 191: 89-95.
Kou, X., Jiang, S., Park, S. J., Meng, L. Y. (2020). A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 49: 6915–6938.
Krysmann M.J., Kelarakis A., Dallas P., Giannelis E.P. (2012). Formation mechanism of carbogenic nanoparticles with dual photoluminescence emission, J. Am. Chem. Soc. 134:747–750.
Li Y., Zhao Y., Cheng H.H., Hu H.H., Shi G.Q., Dai L.M., Qu L.T.(2012). Nitrogen-doped graphene quantum dots with oxygen-rich functional groups, J. Am. Chem. Soc. 134:15–18.
Lin Z., Xue W., Chen H., Lin J.M. (22012). Classical oxidant induced chemiluminescence of fluorescent carbon dots, Chem. Commun. 48(7). 1051e1053. doi:10.1039/c1cc15290d
Liu C., Zhang P., Tian F., Li W., Li F., Liu W. (2011). One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging, J. Mater. Chem. 21: 13163-13167.
Mosconi D., Mazzier D., Silvestrini S., Privitera A., Marega C., Franco L., Moretto A.(2015). Synthesis and photochemical applications of processable polymers enclosing photoluminescent carbon quantum dots, ACS Nano 9: 4156-4164.
Muhammad F.F., Sulaiman K. (2011). Utilizing a simple and reliable method to investigate the optical functions of small molecular organic flms-Alq3 and Gaq3 as examples. Measurement 44:1468–1474.
Mullen W., McGinn J., Lean M.J., MacLean M.R., Gardiner P., Duthie G.G. (2002). Ellagitannins, flavanoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J Agri Food Chem. 50:5191–6.
Nie H., Li M.J., Li Q.S., Liang S.J., Tan Y.Y., Sheng L. (2014). Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric pH Sensing, Chem. Mater. 26(10): 3104–3112. doi.org/10.1021/cm5003669
Pancholi B., Rana A.C. (2020). Traditional Uses, Phytochemistry and Pharmacological Aspects of Rubus niveus thumb Plant – A Review The J.ournal of Phytopharmacology 9(6): 438-444.
Pires N.R., Santos C.M.W., Sousa R.R., de Paula R.C.M., Cunha P.L.R., Feitosa J.P.A. (2015). Novel and fast microwave-assisted synthesis of carbon quantum dots from raw cashew gum, J. Braz. Chem. Soc. 26:1274-1282.
Qu L.T., Liu V, Baek J.B., Dai L.M. (2010). Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells, ACS Nano. 4:1321–1326.
Raja S., Ramesh V., Thivaharan V. (2015). Green biosynthesis of silver nanoparticles using calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability, Arabian J. Chem. DOI: 10.1016/j.arabjc.2015.06.023.
Rooj B., Dutta A., Islam S., Mandal U. (2018). Green Synthesized Carbon Quantum Dots from Polianthes tuberose L. Petals for Copper (II) and Iron (II) Detection, J. Fluoresc 28: 1261–1267.
Salinas-Castillo A., Ariza-Avidad M., Pritz C., Camprubí-Robles M., Fern andez B., Ruedas-Rama M.J., Megia-Fernandez A., Lapresta-Fern andez A., Santoyo-Gonzalez F., Schrott-Fischer A., Capitan-Vallvey L.F. (2013). Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources, Chem. Commun. 49: 1103-1105.
Sathishkumar G., Gobinath C., Karpaga K., Hemamalini V., Premkumar K., Sivaramakrishnan S. (2012). Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens, Colloids Surf., B. 95:235-240.
Shamsipur M., Rajabi H. (2014). Pure zinc sulfide quantum dot as highly selective luminescent probe for determination of hazardous cyanide ion, Mater. Sci. Eng. C 36: 139–145.
Singh R., Dutta S. (2018). Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties, Adv. Powder Technol. 29: 211–219.
Su B.N., Pawlus A.D., Jung H.A., Keller W.J., McLaughlin J.L., Kinghorn A. D. (2005). Chemical Constituents of the Fruits of Morinda citrifolia (Noni) and their antioxidant Activity, J. Nat. Prod. 68:592-595.
Tantama M., Hung Y.P., Yellen G.J.(2011). Imaging Intracellular pH in Live Cells with a Genetically Encoded Red Fluorescent Protein Sensor, Am. Chem. Soc. 133: 10034-10037.
Tauc J., Grigorovici R., Vancu A. (1966). Optical properties and electronic structure of amorphous germanium. Physica status solidi (b), 15:627-637.
Wang H., Sun P., Cong S., Wu J., Gao L., Wang Y., Dai X., Yi Q., Zou G. (2016). Nitrogen-Doped Carbon Dots for “green” Quantum Dot Solar Cells, Nanoscale Res. Lett., 11: 1–6.
Wang X., Qu Q., Xu B., Ren J., Qu X. (2011). Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents, J. Mater. Chem. 21: 2445-2450.
Xiao L., Sun H. (2018). Novel properties and applications of carbon nanodots, Nanoscale Horizons, 3(6): 565–597.
Yakuphanoglu F., Erten H. (2005). Refractive index dispersion and analysis of the optical constants’ of an ionomer thin flm. Opt Appl 35(4):969.
Zhang J., Yu S.-H. (2016). Carbon dots: large-scale synthesis, sensing and bioimaging, Mater. Today 19(7):382–393.
Zyoud A.H., Zubi A., Zyoud S.H., Hilal M.H., Zyoud S., Qamhieh N., Hajamohideen A., Hilal H.S.(2019). Kaolin-supported ZnO nanoparticle catalysts in self-sensitized tetracycline photodegradation: Zero-point charge and pH effects. Appl. Clay Sci. 182, 105294. doi:10.1016/j.clay.2019.105294